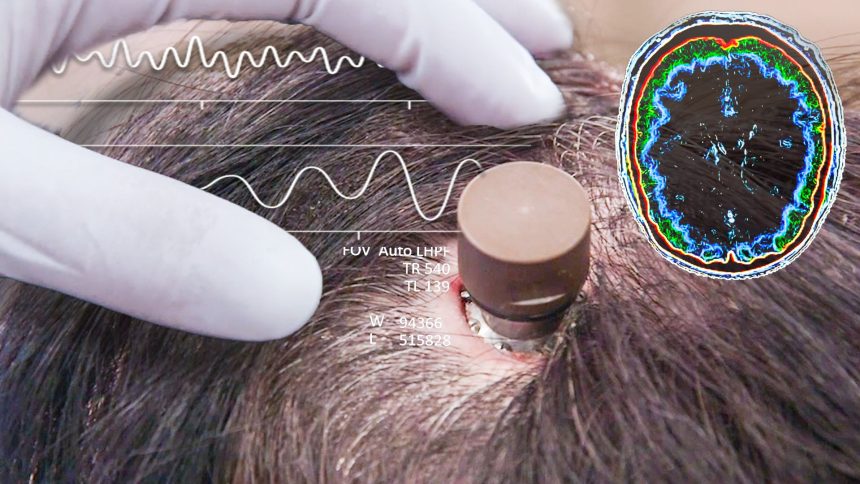
Neuralink, the brain-implant company founded by Elon Musk, has achieved a groundbreaking milestone as it secures approval from the U.S. Food and Drug Administration (FDA) to launch its first-in-human clinical trials.
After facing initial setbacks, this achievement marks a significant step forward for Neuralink and its mission to revolutionize the field of neurology and empower individuals with neurological disorders.
Elon Musk has long been optimistic about Neuralink’s potential, predicting FDA approval for human trials since 2019. However, the company faced a setback in early 2022 when its initial application was rejected. Determined to address the FDA’s safety concerns regarding the experimental implant, Neuralink diligently worked through the agency’s requirements, culminating in the recent approval.
“This is the result of incredible work by the Neuralink team in close collaboration with the FDA and represents an important first step that will one day allow our technology to help many people,” Neuralink expressed in a tweet, acknowledging the monumental efforts put forth by its dedicated team.
We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study!
This is the result of incredible work by the Neuralink team in close collaboration with the FDA and represents an important first step that will one day allow our…
— Neuralink (@neuralink) May 25, 2023
Neuralink’s ambitious mission centers around the development of a revolutionary brain implant that has the potential to restore mobility to paralyzed individuals and address a myriad of neurological ailments. The technology aims to establish a direct connection between the human brain and external devices, leveraging advanced neural interfaces and artificial intelligence.
The envisioned impact of Neuralink’s work is profound. Through brain-computer interface technology, the company aims to enable individuals with paralysis to regain mobility by controlling prosthetic limbs or exoskeletons using their own thoughts. This pioneering approach has the power to transform the lives of millions worldwide, instilling hope and offering unprecedented possibilities for those with spinal cord injuries or other debilitating neurological conditions.
Neuralink’s brain implants consist of ultra-thin threads, finer than a human hair, that can be implanted directly into the brain. These threads are equipped with electrodes capable of detecting and stimulating neural activity, facilitating bidirectional communication between the brain and external devices. The data collected from the brain can then be analyzed and interpreted using advanced algorithms and machine learning, propelling our understanding of brain function and paving the way for personalized treatments.
While Neuralink has received FDA approval for the clinical study, it is important to note that the company has not yet commenced participant recruitment for the trial. This signifies that further preparations and protocols need to be established before the study can proceed.
Neuralink may encounter several challenges in recruiting suitable participants, such as:
Ethical Considerations: Conducting a clinical study involving brain implants raises complex ethical considerations. Neuralink must ensure that participants fully understand the risks and benefits associated with the procedure, as well as any potential long-term effects. They must also prioritize informed consent and ensure that participants are aware of the experimental nature of the technology.
Participant Eligibility: Neuralink will need to define specific criteria for participant eligibility based on the goals and focus of their study. They may face challenges in finding individuals who meet the specific requirements, such as those with the appropriate neurological conditions or individuals who are willing to undergo an invasive procedure. Additionally, finding participants who are committed to long-term follow-up and data collection may pose a challenge.
Safety Concerns: Given the innovative nature of Neuralink’s technology, safety concerns will be paramount. Potential participants may have reservations about the risks associated with the brain implant procedure and its long-term effects. Ensuring participant safety and providing comprehensive information about the safety measures in place will be crucial to address any concerns.
Trust and Public Perception: Neuralink’s success in recruiting participants may depend on establishing trust and addressing public perception. The concept of brain implants can be met with skepticism and fear. Neuralink must effectively communicate the potential benefits, safety precautions, and transparently address any concerns raised by the public and potential participants.
Limited Accessibility: Depending on the geographic location of the clinical study, Neuralink may face challenges in reaching a diverse and representative participant pool. Accessibility barriers, such as travel distance and financial constraints, could limit the participation of individuals from different backgrounds or regions.
However, these challenges can be overcome if Neuralink can employ strategies such as collaborating with reputable medical institutions, engaging with patient advocacy groups, and implementing comprehensive participant education and support programs.
By demonstrating transparency, prioritizing participant safety, and addressing ethical concerns, the brain-implant company can enhance its chances of successfully recruiting participants for its groundbreaking human clinical study.
While the exact timeline for Neuralink’s advancements and the widespread availability of its technology remain uncertain, the FDA’s approval for the human clinical study marks a significant leap forward. It underscores the potential of Neuralink’s approach and sets the stage for further research and development in the field of neural interfaces.
As the company progresses, it will need to conduct rigorous human clinical trials to ensure safety and efficacy while addressing any challenges that may arise. With each step forward, Neuralink is poised to unlock invaluable insights into the workings of the human brain, potentially leading to new treatment avenues for neurological ailments and expanding our understanding of cognition and consciousness.